Powering the Cell: Cellular Respiration and Glycolysis
Article objectives
You know that humans deprived of oxygen for more than a few minutes will quickly become unconscious and die. Breathing, also known as respiration, is essential for human life, because the body cannot store oxygen for later use as it does food. The mammalian respiratory system, shown in Figure 1 features a diaphragm, trachea, and a thin membrane whose surface area is equivalent to the size of a handball court - all for efficient oxygen intake. Other forms of life employ different types of respiratory organs: fish and aquatic amphibians and insects flaunt gills, spiders and scorpions develop ”book lungs,” and terrestrial insects use an elaborate network of tubes called tracheae, which open via spiracles, as shown in Figure 2 and Figure 3. A constant supply of oxygen gas is clearly important to life. However, do you know why you need oxygen?

Figure 1: The human respiratory system is only part of the story of respiration. Diaphragm, lungs, and trachea take air deep into the body and provide oxygen gas to the bloodstream. The fate of that oxygen is the story of cellular respiration.
Many people would answer that oxygen is needed to make carbon dioxide, the gas exhaled or released by each of the respiratory systems listed above. However, \(CO_2\) is waste product. Surely, there is more to the story than just gas exchange with the environment! To begin to appreciate the role of oxygen inside your body, think about when your breathing rate increases: climbing a steep slope, running a race, or skating a shift in a hockey game. Respiration rate correlates with energy use, and that correlation reflects the link between oxygen and energy metabolism. For this reason, the chemical reactions inside your cells that consume oxygen to produce usable energy are known as cellular respiration.

Figure 2: Spiracles in this Indian Luna Moth (Actias selene) caterpillar connect to a system of internal tubes (tracheae) which carry oxygen throughout the animal’s body.

Figure 3: Gills in this alpine newt larva, Triturus alpestris, bring blood close to an extensive surface area so that the newt can absorb dissolved oxygen gas from its watery habitat.
An Overview of Cellular Respiration
Another way to think about the role of oxygen in your body - and a good starting point for understanding the whole process of cellular respiration - is to recall the last time you sat by a campfire (see below figure) and noticed that it was ”dying.” Often people will blow on a campfire to keep it from ”dying out.” How does blowing help? What happens in a campfire?

Figure 4: Analyzing what happens when wood burns in a campfire is a good way to begin to understand cellular respiration.
You know that a fire produces light and heat energy. However, it cannot ”create” energy (remember that energy cannot be created or destroyed). Fire merely transforms the energy stored in its fuel – chemical energy – into light and heat. Another way to describe this energy transformation is to say that burning releases the energy stored in fuel. As energy is transformed, so are the compounds that make up the fuel. In other words, burning is a chemical reaction. We could write our understanding of this energy-releasing chemical reaction up to this point as:
$$\stackrel{\hbox{Wood Fuel}}{{\scriptsize \text{Stored chemical energy}}} \xrightarrow{\text{Spark or match}} \text{light energy} + \text{heat energy}$$
Now return to what happens when you blow on a fire. The fire was ”dying out,” so you blew on it to get it going again. Was it movement or something in the air that promoted the chemical reaction? If you have ever ”smothered” a fire, you know that a fire needs something in the air to keep burning. That something turns out to be oxygen. Oxygen gas is a reactant in the burning process. At this point, our equation is:
$$O_2 + \stackrel{\hbox{Wood Fuel}}{{\scriptsize \text{Stored chemical energy}}} \xrightarrow{\text{Spark or match}} \text{light energy} + \text{heat energy}$$
To complete this equation, we need to know what happens to matter, to the atoms of oxygen, and to the atoms of the fuel during the burning. If you collect the gas rising above a piece of burning wood in an inverted test tube, you will notice condensation - droplets appearing on the sides of the tube. Cobalt chloride paper will change from blue to pink, confirming that these droplets are water. If you add bromothymol blue (BTB) to a second tube of collected gases, the blue solution will change to green or yellow (Figure 5), indicating the presence of carbon dioxide. Thus, carbon dioxide and water are products of burning wood.

Figure 5: Bromothymol blue (BTB) changes from blue to green to yellow as carbon dioxide is added. Thus, it is a good indicator for this product of burning or cellular respiration.
$$O_2 + \stackrel{\hbox{Wood Fuel}}{{\scriptsize \text{Stored chemical energy}}} \xrightarrow{\text{Spark or match}} CO_2 + H_2 O + \text{light energy} + \text{heat energy}$$
Now we know what happened to those oxygen atoms during the chemical reaction, but we need to be sure to identify the sources of the carbon atoms in the \(CO_2\) and of the hydrogen atoms in the water. If you guessed that these atoms make up the wood fuel – and nearlyall fuels we burn, from coal to propane to candle wax to gasoline (hydrocarbons!), you have solved the equation completely. Overall, burning is the combining of oxygen with hydrogen and carbon atoms in a fuel (combustion or oxidation) to release the stored chemical energy as heat and light. Products of combustion are \(CO_2\) (oxidized carbon) and \(C_2 O\) (oxidized hydrogen). Or in symbols,
$$O_2 + \stackrel{\hbox{C}_x-\hbox{H}_y}{{\scriptsize \text{Stored chemical energy}}} \xrightarrow{\text{Spark or match}} CO_2 + H_2 O + \text{light energy} + \text{heat energy}$$
Return to the fate of the oxygen gas you breathe in and absorb. Recall that we related breathing rate and oxygen intake to energy use. Burning consumes oxygen as it releases stored chemical energy, transforming it into light and heat. Cellular respiration is actually a slow burn. Your cells absorb the oxygen carried by your blood from your lungs, and use the \(O_2\) to release stored chemical energy so that you can use it.
However, releasing energy within cells does not produce light or intense heat. Cells run on chemical energy – specifically, the small amount temporarily stored in adenine triphosphate (ATP) molecules. Cellular respiration transfers chemical energy from a ”deliverable” fuel molecule – glucose – to many ”usable” molecules of ATP. Like oxygen, glucose is delivered by your blood to your cells. If ATP were delivered to cells, more than 60,221,417,930,000,000,000,000,000 of these large molecules (which contain relatively small amounts of energy) would clog your capillaries each day. Pumping them across cell membranes would ”cost” a great deal of energy. A molecule of glucose contains a larger amount of chemical energy in a smaller package. Therefore, glucose is much more convenient for bloodstream delivery, but too ”powerful” to work within the cell. The process of cellular respiration uses oxygen to help transfer the chemical energy from glucose to ATP, which can be used to do work in the cell. This chemical equation expresses what we have worked out:
$$O_2 + \stackrel{\hbox{C}_6 \hbox{H}_12 \hbox{O}_6}{{\scriptsize \text{Deliverable stored chemical energy}}} \longrightarrow \stackrel{\hbox{ATP}}{{\scriptsize \text{Usable stored energy}}}$$
As with burning, we must trace what happens to atoms during cellular respiration. You can readily see that when the carbon atoms in glucose are combined with oxygen, they again form carbon dioxide. And when the hydrogen atoms in glucose are oxidized, they form water, as in burning. You can detect these products of cellular respiration in your breath on a cold day (as water condensation) and in the lab (BTB turns yellow when you blow into it through a straw). The equation:
$$O_2 + \stackrel{\hbox{C}_6 \hbox{H}_12 \hbox{O}_6}{{\scriptsize \text{Deliverable stored chemical energy}}} \longrightarrow \stackrel{\hbox{ATP}}{{\scriptsize \text{Usable stored energy}}} + CO_2 + H_2 O$$
This accounts for the energy transfer and the carbon, hydrogen, and oxygen atoms, but it does not show the ”raw materials” or reactants which build ATP. Recall that the energy temporarily stored in ATP is released for use when the bond between the second and third phosphates is broken. The resulting ADP can be recycled within the cell by recombining it with inorganic phosphate (\(P_i\)).
Now you should be able to see that the source of energy for re-attaching the phosphate is the chemical energy in glucose! Materials cycle and recycle, but energy gets used up and must be replaced. That is the key to understanding cellular respiration: it is a ”recharging of the batteries” - ATP molecules – which power cellular work. How many ATP can be made by harnessing the energy in a single glucose molecule? Although this number varies under certain conditions, most cells can capture enough energy from one molecule of glucose to build 38 molecules of ATP. Our equation becomes:
$$O_2 + \stackrel{\hbox{C}_6 \hbox{H}_12 \hbox{O}_6}{{\scriptsize \text{Deliverable stored chemical energy}}} + 38\text{ADP} + 38\text{P}_i \longrightarrow \stackrel{\hbox{38ATP}}{{\scriptsize \text{Usable stored energy}}} + CO_2 + H_2 O$$
This equation for cellular respiration is not quite complete, however, because we can easily mix air and glucose sugar (even adding ADP and Pi) and nothing will happen. For the campfire, we indicated above the arrow that a necessary condition was a spark or match to start the reaction. A spark or match would damage or destroy living tissue. What necessary condition initiates the slow burn that is cellular respiration? Recall that enzymes are highly specific proteins which ”speed up” chemical reactions in living cells. More than 20 kinds of enzymes carry out cellular respiration! If you also recall that membranes within organelles often sequence enzymes for efficiency, as in chloroplasts for photosynthesis, you will not be surprised that a specific organelle, the mitochondrion (Figure 6), is also a necessary condition of cellular respiration - at least in eukaryotes.

Figure 6: Mitochondria are membranous organelles which sequence enzyme and electron carrier molecules to make cellular respiration highly efficient.
Within each eukaryotic cell, the membranes of 1000-2000 mitochondria sequence enzymes and electron carriers and compartmentalize ions so that cellular respiration proceeds efficiently. Mitochondria, like chloroplasts, contain their own DNA and ribosomes and resemble certain bacteria. The endosymbiotic theory holds that mitochondria, too, were once independently living prokaryotes. Larger prokaryotes engulfed (or enslaved) these smaller aerobic cells, forming eukaryotic cells. Many prokaryotes today can perform cellular respiration; perhaps they and mitochondria have common ancestors. Their expertise in generating ATP made mitochondria highly valued symbionts.
Including these necessary conditions and balancing numbers of atoms on both sides of the arrow, our final equation for the overall process of cellular respiration is:
$$6O_2 + \stackrel{\hbox{C}_6 \hbox{H}_12 \hbox{O}_6}{{\scriptsize \text{Deliverable stored chemical energy}}} + 38\text{ADP} + 38\text{P}_i \xrightarrow{\stackrel{\text{mitochondria}}{\text{enzymes}}} \stackrel{\hbox{38ATP}}{{\scriptsize \text{Usable stored energy}}} + 6CO_2 + 6H_2 O$$
In words, cellular respiration uses oxygen gas to break apart the carbon-hydrogen bonds in glucose and release their energy to build 38 molecules of ATP. Most of this process occurs within the mitochondria of the cell. Carbon dioxide and water are waste products. This is similar to burning, in which oxygen breaks the carbon-hydrogen bonds in a fuel and releases their chemical energy as heat and light. Again, carbon dioxide and water are waste.
If you have studied the process of photosynthesis, you’ve probably already noticed its similarity to the process of cellular respiration. Both are processes within the cell which make chemical energy available for life. Photosynthesis transforms light energy into chemical energy stored in glucose, and cellular respiration releases the energy from glucose to build ATP, which does the work of life. Moreover, photosynthesis reactants \(CO_2\) and \(H_2 O\) are products of cellular respiration. And the reactants of respiration, \(C_6 H_{12} O_6\) and \(O_2\), are the products of photosynthesis. This interdependence is the basis of the carbon-oxygen cycle (Figure 7), which connects producers to consumers and their environment. At first glance, the cycle merely seems to show mitochondria undoing what chloroplasts do; but the cycle’s energy transformations power all the diversity, beauty, and mystery of life.

Figure 7: Photosynthesis in the chloroplast and cellular respiration in the mitochondrion show the interdependence of producers and consumers, the flow of energy from sunlight to heat, and the cycling of carbon and oxygen between living world and environment.
Glycolysis: A Universal and Ancient Pathway for Making ATP
When was the last time you enjoyed yogurt on your breakfast cereal, or had a tetanus shot? These experiences may appear unconnected, but both relate to bacteria which do not use oxygen to make ATP. In fact, tetanus bacteria cannot survive if oxygen is present. However, Lactobacillus acidophilus (bacteria which make yogurt) and Clostridium tetani (bacteria which cause tetanus or lockjaw) share with nearly all organisms the first stage of cellular respiration, glycolysis (Figure 8). Because glycolysis is universal, whereas aerobic (oxygen-requiring) cellular respiration is not, most biologists consider it to be the most fundamental and primitive pathway for making ATP.

Figure 8: Clostridium tetani bacteria are obligate anaerobes, which cannot grow in the presence of oxygen and use a variation of glycolysis to make ATP. Because they can grow in deep puncture wounds and secrete a toxin, which can cause muscle spasms, seizures, and death, most people receive tetanus vaccinations at least every ten years throughout life.
Return to the overall equation for cellular respiration:
$$6O_2 + \stackrel{\hbox{C}_6 \hbox{H}_12 \hbox{O}_6}{{\scriptsize \text{Deliverable stored chemical energy}}} + 38\text{ADP} + 38\text{P}_i \xrightarrow{\stackrel{\text{enzymes}}{\text{mitochondria}}} \stackrel{\hbox{38ATP}}{{\scriptsize \text{Usable stored energy}}} + 6CO_2 + 6H_2 O$$
Like photosynthesis, the process represented by this equation is actually many small, individual chemical reactions. We grouped the reactions of photosynthesis into two stages, the light reactions and the Calvin Cycle. We will divide the reactions of cellular respiration into three stages: glycolysis, the Krebs Cycle, and the electron transport chain (Figure 9). We will explore Stage 1, glycolysis - the oldest and most widespread pathway for making ATP. Before diving into the details, we must note that this first stage of cellular respiration is unique among the three stages: it does not require oxygen, and it does not take place in the mitochondrion. The chemical reactions of glycolysis occur without oxygen in the cytosol of the cell (Figure 10).
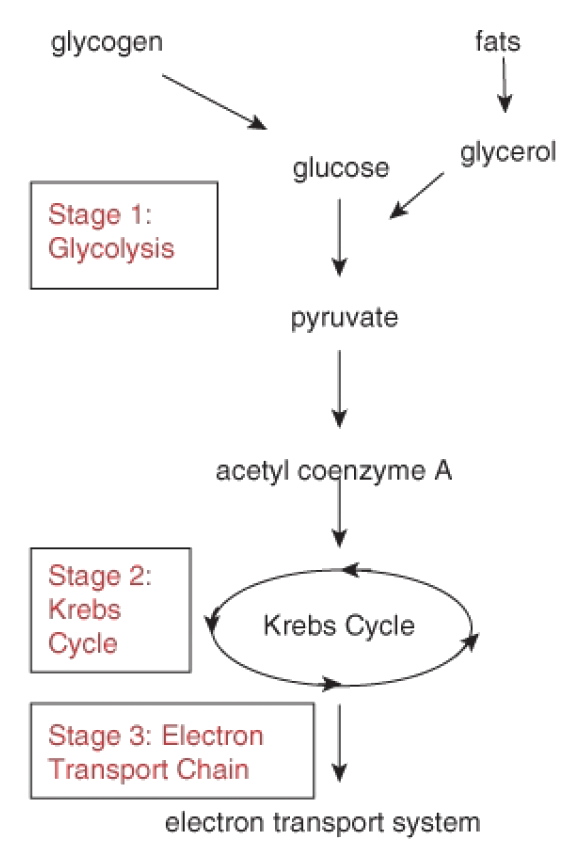
Figure 9: The many steps in the process of aerobic cellular respiration can be divided into three stages. The first stage, glycolysis, produces ATP without oxygen. Because this part of the cellular respiration pathway is universal, biologists consider it the oldest segment. Note that glycogen and fats can also enter the glycolysis pathway.

Figure 10: Glycolysis, unlike the latter two stages of cellular respiration, takes place without oxygen in the cytosol of the cell. For many organisms, aerobic respiration continues with the Krebs cycle and the electron transport chain in the mitochondria.
The name for Stage 1 clearly indicates what happens during that stage: glyco- refers to glucose, and -lysis means ”splitting.” In glycolysis, within the cytosol of the cell, a minimum of eight different enzymes break apart glucose into two 3-carbon molecules. The energy released in breaking those bonds is transferred to carrier molecules, ATP and NADH. NADH temporarily holds small amounts of energy which can be used later to build ATP. The 3- carbon product of glycolysis is pyruvate, or pyruvic acid (Figure 11). Overall, glycolysis can be represented as shown below:
$$C_6 H_{12} O_6 + 2NAD^+ + 2P_i + 2ADP \longrightarrow 2\text{pyruvate} + 2 NADH + 2ATP$$

Figure 11: Glycolysis breaks the 6-carbon molecule glucose into two 3-carbon pyruvate molecules, releasing some of the chemical energy which had been stored in glucose.
However, even this equation is deceiving. Just the splitting of glucose requires many steps, each transferring or capturing small amounts of energy. Individual steps appear in Figure 12. Studying the pathway in detail reveals that cells must ”spend” or ”invest” two ATP in order to begin the process of breaking glucose apart. Note that the phosphates produced by breaking apart ATP join with glucose, making it unstable and more likely to break apart. Later steps harness the energy released when glucose splits, and use it to build ”hot hydrogens” (\(NAD^+\) is reduced to NADH) and ATP (\(ADP + P_i \rightarrow ATP\)). If you count the ATP produced, you will find a net yield of two ATP per glucose (4 produced – 2 spent). Remember to double the second set of reactions to account for the two 3 carbon molecules which follow that pathway! The ”hot hydrogens” can power other metabolic pathways, or in many organisms, provide energy for further ATP synthesis.

Figure 12: This detailed diagram demonstrates that glycolysis ”costs” 2 ATP, but harnesses enough energy from breaking bonds in glucose to produce 4 ATP and 2 pairs of ”hot hydrogens” (\(NADH + H^+\)). Note the multiplier (2X) required for the 3-carbon steps.
To summarize: In the cytosol of the cell, glycolysis transfers some of the chemical energy stored in one molecule of glucose to two molecules of ATP and two NADH. This makes (some of) the energy in glucose, a universal fuel molecule for cells, available to use in cellular work - moving organelles, transporting molecules across membranes, or building large organic molecules.
Although glycolysis is universal, pathways leading away from glycolysis vary among species depending on the availability of oxygen. If oxygen is unavailable, pyruvate may be converted to lactic acid or ethanol and carbon dioxide in order to regenerate \(NAD^+\), ending anaerobic respiration. Anaerobic respiration is also called fermentation.
If oxygen is present, pyruvate enters the mitochondria for further breakdown, releasing far more energy and producing many more molecules of ATP in the latter two stages of aerobic respiration - the Krebs cycle and electron transport chain.
Images courtesy of:
CK-12 Foundation. http://pam.wikipedia.org/wiki/File:3DScience_respiratory_labeled.jpg. CC-BY-SA.
Shawn Hanrahan. http://commons.wikimedia.org/wiki/Image: Actias_selene_5th_instar_spiracles_sjh.jpg. CC-BY-SA 2.5.
André Karwath. http://commons.wikimedia.org/wiki/Image:Smooth_Newt_larva_%28aka%29.jpg. CC-BY-SA 2.5.
Túrelio (http://commons.wikimedia.org/wiki/User:T%C3%BArelio). http://commons.wikimedia.org/wiki/Image:HolzfeuerFlussbett.jpg. CC-BY-SA 2.5.
Brandon Fesser. http://commons.wikimedia.org/wiki/Image:Bromothymol_blue_colors.jpg. Public Domain.
CK-12 Foundation,NIH. http://commons.wikimedia.org/wiki/Image:MitoChondria.jpg. Public Domain.
CK-12 Foundation. http://commons.wikimedia.org/wiki/File:Chloroplast.png. Public Domain,GNU-FDL.
CDC. http://commons.wikimedia.org/wiki/Image:Clostridium_tetani_01.png. Public Domain.
CK-12 Foundation. http://commons.wikimedia.org/wiki/Image: Cellular_respiration_flowchart_%28en%29.svg. Public Domain.
CK-12 Foundation,MesserWoland and Szczepan. http://commons.wikimedia.org/wiki/Image:Biological_cell.svg. GNU-FDL.
CK-12 Foundation,Rob Hooft. http://commons.wikimedia.org/wiki/Image:Alpha-d-glucose.png. GNU-FDL.
Rozzychan. http://commons.wikimedia.org/wiki/Image:GlycolysiscompleteLabelled.png. Public Domain.