Energy for Life: An Overview of Photosynthesis
Article objectives
All living things require an ongoing source of energy to do the work of life. You often see energy in action on a large scale: a whale breaches, apple blossoms swell and burst, a firefly glows, or an inky cap mushrooms overnight. However, energy works constantly to maintain life on a very small scale as well. Inside each cell of every organism, energy assembles chains of information and constructs cellular architecture. It moves tiny charged particles and giant protein molecules. Moreover, it builds and powers cell systems for awareness, response, and reproduction. All life’s work requires energy.
Physics tells us that organized systems, such as living organisms, tend to disorder without a constant input of energy. You have direct, everyday experience with this law of nature: after a week of living in your room, you must spend energy in order to return it to its previous, ordered state. Tides and rain erode your sandcastles, so you must work to rebuild them. And your body, after a long hike or big game, must have more fuel to keep going. Living things show amazing complexity and intricate beauty, but if their source of energy fails, they suffer injury, illness, and eventually death.
Physics also tells us that, although energy can be captured or transformed, it inevitably degrades, becoming heat, a less useful form of energy. This is why organisms require a constant input of energy; the work they must do uses up the energy they take in. Energy, unlike materials, cannot be recycled. The story of life is a story of energy flow – its capture, transformation, use for work, and loss as heat.
Energy, the ability to do work, can take many forms: heat, nuclear, electrical, magnetic, light, and chemical energy. Life runs on chemical energy - the energy stored in covalent bonds between atoms in a molecule. Where do organisms get their chemical energy? That depends…
How Do Organisms Get Energy? Autotrophs vs. Heterotrophs
Living organisms obtain chemical energy in one of two ways.
Autotrophs, shown in Figure 1, store chemical energy in carbohydrate food molecules they build themselves. Food is chemical energy stored in organic molecules. Food provides both the energy to do work and the carbon to build bodies. Because most autotrophs transform sunlight to make food, we call the process they use photosynthesis. Only three groups of organisms - plants, algae, and some bacteria - are capable of this life-giving energy transformation. Autotrophs make food for their own use, but they make enough to support other life as well. Almost all other organisms depend absolutely on these three groups for the food they produce. The producers, as autotrophs are also known, begin food chains which feed all life.

Figure 1: Photosynthetic autotrophs, which make food for more than 99% of the organisms on earth, include only three groups of organisms: plants such as the redwood tree (a), algae such as kelp (b), and certain bacteria like this Anabaena (c).
Heterotrophs cannot make their own food, so they must eat or absorb it. For this reason, heterotrophs are also known as consumers. Consumers include all animals and fungi and many protists and bacteria. They may consume autotrophs, or other heterotrophs or organic molecules from other organisms. Heterotrophs show great diversity and may appear far more fascinating than producers. But heterotrophs are limited by our utter dependence on those autotrophs which originally made our food. If plants, algae, and autotrophic bacteria vanished from earth, animals, fungi, and other heterotrophs would soon disappear as well. All life requires a constant input of energy. Only autotrophs can transform that ultimate, solar source into the chemical energy in food which powers life, as shown in Figure 2.

Figure 2: Food chains carry energy from producers (autotrophs) to consumers (heterotrophs). 99 percent of energy for life comes from the sun via photosynthesis. Note that only nutrients recycle. Energy must continue to flow into the system.
Photosynthesis provides over 99 percent of the energy supply for life on earth. A much smaller group of autotrophs - mostly bacteria in dark or low-oxygen environments - produce food using the chemical energy stored in inorganic molecules such as hydrogen sulfide, ammonia, or methane. While photosynthesis transforms light energy to chemical energy, this alternate method of making food transfers chemical energy from inorganic to organic molecules. It is therefore called chemosynthesis, and is characteristic of the tubeworms shown in Figure 3. Some of the most recently discovered chemosynthetic bacteria inhabit deep ocean hot water vents or “black smokers.” There, they use the energy in gases from the Earth’s interior to produce food for a variety of unique heterotrophs: giant tube worms, blind shrimp, giant white crabs, and armored snails. Some scientists think that chemosynthesis may support life below the surface of Mars, Jupiter’s moon, Europa, and other planets as well. Ecosystems based on chemosynthesis may seem rare and exotic, but they too illustrate the absolute dependence of heterotrophs on autotrophs for food.

Figure 3: Tubeworms deep in the Gulf of Mexico get their energy from chemosynthetic bacteria living within their tissues. No digestive systems needed! Photo: Charles Fisher
Food and Other Energy-Carrying Molecules
You know that the fish you had for lunch contained protein molecules. But do you know that the atoms in that protein could easily have formed the color in a dragonfly’s eye, the heart of a water flea, and the whiplike tail of a Euglena before they hit your plate as sleek fish muscle? As you learned above, food consists of organic (carbon-containing) molecules which store energy in the chemical bonds between their atoms. Organisms use the atoms of food molecules to build larger organic molecules including proteins, DNA, and fats and use the energy in food to power life processes. By breaking the bonds in food molecules, cells release energy to build new compounds. Although some energy dissipates as heat at each energy transfer, much of it is stored in the newly made molecules. Chemical bonds in organic molecules are a reservoir of the energy used to make them. Fueled by the energy from food molecules, cells can combine and recombine the elements of life to form thousands of different molecules. Both the energy (despite some loss) and the materials (despite being reorganized) pass from producer to consumer – perhaps from algal tails, to water flea hearts, to dragonfly eye colors, to fish muscle, to you!
The process of photosynthesis, which usually begins the flow of energy through life, uses many different kinds of energy-carrying molecules to transform sunlight energy into chemical energy and build food.
Some carrier molecules hold energy briefly, quickly shifting it like a hot potato to other molecules. This strategy allows energy to be released in small, controlled amounts. An example is chlorophyll, the green pigment present in most plants which helps convert solar energy to chemical energy. When a chlorophyll molecule absorbs light energy, electrons are excited and “jump” to a higher energy level. The excited electrons then bounce to a series of carrier molecules, losing a little energy at each step. Most of the “lost” energy powers some small cellular task, such as moving ions across a membrane or building up another molecule. Another short-term energy carrier important to photosynthesis, NADPH, holds chemical energy a bit longer but soon “spends” it to help to build sugar.
Two of the most important energy-carrying molecules are glucose and ATP, adenosine triphosphate. These are nearly universal fuels throughout the living world and both are also key players in photosynthesis, as shown below.
A molecule of glucose, which has the chemical formula C6H12O6, carries a packet of chemical energy just the right size for transport and uptake by cells. In your body, glucose is the “deliverable” form of energy, carried in your blood through capillaries to each of your 100 trillion cells. Glucose is also the carbohydrate produced by photosynthesis, and as such is the near-universal food for life.
ATP molecules store smaller quantities of energy, but each releases just the right amount to actually do work within a cell. Muscle cell proteins, for example, pull each other with the energy released when bonds in ATP break open. The process of photosynthesis also makes and uses ATP - for energy to build glucose! ATP, then, is the useable form of energy for your cells.
Glucose is the energy-rich product of photosynthesis, a universal food for life. It is also the primary form in which your bloodstream delivers energy to every cell in your body. The six carbons are numbered.
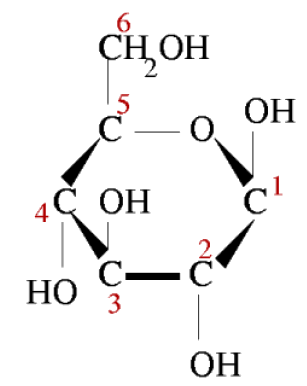
Figure 4: Glucose
Why do we need both glucose and ATP? Why don’t plants just make ATP and be done with it? If energy were money, ATP would be a quarter. Enough money to operate a parking meter or washing machine. Glucose would be a dollar bill (or $10) – much easier to carry around in your wallet, but too large to do the actual work of paying for parking or washing. Just as we find several denominations of money useful, organisms need several “denominations” of energy – a smaller quantity for work within cells, and a larger quantity for stable storage, transport, and delivery to cells.
Let’s take a closer look at a molecule of ATP. Although it carries less energy than glucose, its structure is more complex. “A” in ATP refers to the majority of the molecule – adenosine – a combination of a nitrogenous base and a five-carbon sugar. “T” and “P” indicate the three phosphates, linked by bonds which hold the energy actually used by cells. Usually, only the outermost bond breaks to release or spend energy for cellular work.
An ATP molecule, shown below, is like a rechargeable battery: its energy can be used by the cell when it breaks apart into ADP (adenosine diphosphate) and phosphate, and then the “worn-out battery” ADP can be recharged using new energy to attach a new phosphate and rebuild ATP. The materials are recyclable, but recall that energy is not! How much energy does it cost to do your body’s work? A single cell uses about 10 million ATP molecules per second, and recycles all of its ATP molecules about every 20-30 seconds.
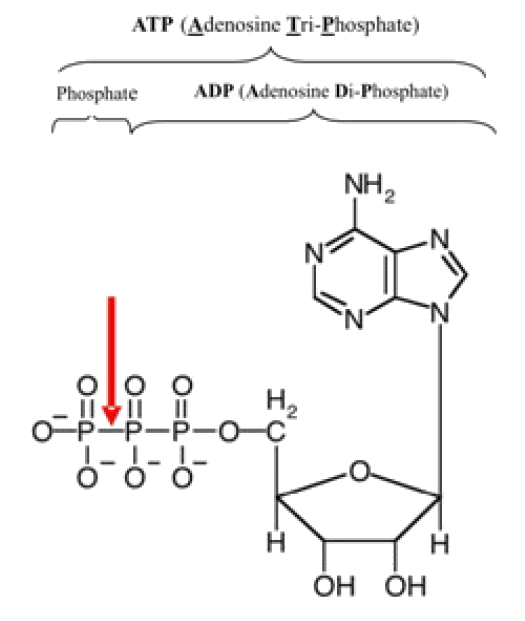
Figure 5: A red arrow shows the bond between two phosphate groups in an ATP molecule. When this bond breaks, its chemical energy can do cellular work. The resulting ADP molecule is recycled when new energy attaches another phosphate, rebuilding ATP.
Photosynthesis: The Most Important Chemical Reaction for Life on Earth
What do pizza, campfires, dolphins, automobiles, and glaciers have in common? Photosynthesis is often considered the most important chemical reaction for life on earth. Let’s delve into how this process works and why we are so indebted to it.
Photosynthesis involves a complex series of chemical reactions, each of which convert one substance to another. These reactions taken as a whole can be summarized in a single symbolic representation – as shown in the chemical equation below.
$$6CO_2 + 6H_2 O + \text{light} \xrightarrow{\stackrel{\text{Chloroplast, Chlorophyll}}{\text{Enzymes}}} C_6 H_{12} O_6 + 6O_2$$
We can substitute words for the chemical symbols. Then the equation appears as below.
$$\text{Carbon dioxide + water + light energy} \xrightarrow{\stackrel{\text{Chloroplast, Chlorophyll}}{\text{Enzymes}}} \text{glucose + oxygen gas}$$
Like all chemical equations, this equation for photosynthesis shows reactants connected by plus signs on the left and products, also connected by plus signs, on the right. An arrow indicating the process or chemical change leads from the reactants to the products, and conditions necessary for the chemical reaction are written above the arrow. Note that the same kinds of atoms, and number of atoms, are found on both sides of the equation, but the kinds of compounds they form change.
You use chemical reactions every time you cook or bake. You add together ingredients (the reactants), place them in specific conditions (often heat), and enjoy the results (the products). A recipe for chocolate chip cookies written in chemical equation form is shown below.
| $$\underbrace{\text{Butter + sugar + eggs + flour + chocolate chips}}_{\text{Reactants}}$$ | $$\underbrace{\xrightarrow{375 ^\circ F, \; 6-10 \; \text{minutes}}}_{\text{Necessary Conditions}}$$ | $$\underbrace{\text{chocolate chip cookies}}_{\text{Product}}$$ |
Compare this familiar recipe to photosynthesis below.
$$6CO_2 + 6H_2 O + \text{light} \xrightarrow{\stackrel{\text{Chloroplast, Chlorophyll}}{\text{Enzymes}}} C_6 H_{12} O_6 + 6O_2$$
The equation shows that the “ingredients” for photosynthesis are carbon dioxide, water, and light energy. Plants, algae, and photosynthetic bacteria take in light from the sun, molecules of carbon dioxide from the air, and water molecules from their environment and combine these reactants to produce food (glucose).
Of course, light, carbon dioxide, and water mix in the air even without plants. But they do not chemically change to make food without very specific necessary conditions which are found only in the cells of photosynthetic organisms. Necessary conditions include:
-
enzymes - proteins which speed up chemical reactions without the heat required for cooking.
-
chlorophyll - a pigment which absorbs light
-
chloroplasts - organelles whose membranes embed chlorophyll, accessory pigments, and enzymes in patterns which maximize photosynthesis
Within plant cells or algal cells, chloroplasts organize the enzymes, chlorophyll, and accessory pigment molecules necessary for photosynthesis.

Figure 6
When the reactants meet inside chloroplasts, or the very similar cells of blue-green bacteria, chemical reactions combine them to form two products: energy-rich glucose molecules and molecules of oxygen gas. Photosynthetic organisms store the glucose (usually as starch) and release the oxygen gas into the atmosphere as waste.
Let’s review the chemical equation for photosynthesis once more, this time at the level of atoms as in the equation below.
$$6CO_2 + 6H_2 O + \text{light} \xrightarrow{\stackrel{\text{Chloroplast, Chlorophyll}}{\text{Enzymes}}} C_6 H_{12} O_6 + 6O_2$$
Look closely at its primary purpose: storing energy in the chemical bonds of food molecules. The source of energy for food is sunlight energy. The source of carbon atoms for the food molecules is carbon dioxide from the air, and the source of hydrogen atoms is water. Inside the cells of plants, algae, and photosynthetic bacteria, chlorophyll, and enzymes use the light energy to rearrange the atoms of the reactants to form the products, molecules of glucose and oxygen gas. Light energy is thus transformed into chemical energy, stored in the bonds which bind six atoms each of carbon and oxygen to twelve atoms of hydrogen – forming a molecule of glucose. This energy rich carbohydrate molecule becomes food for the plants, algae, and bacteria themselves as well as for the heterotrophs which feed on them.
One last detail: why do “6”s precede the \(CO_2, H_2 O\), and \(O_2\)? Look carefully, and you will see that this “balances” the equation: the numbers of each kind of atom on each side of the arrow are equal. Six molecules each of \(CO_2\) and \(H_2 O\) make 1 molecule of glucose and 6 molecules of oxygen gas.
Images courtesy of:
http://commons.wikimedia.org/wiki/File:Kelp_300.jpg http://upload.wikimedia.org/wikipedia/commons/6/64/Anabaena_sperica.jpeg. (a)Public Domain (b)Public Domain (c)Creative Commons.
CK-12 Foundation. CC-BY-SA.
Antje Boetius. http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0030102. CC-BY.