Human Chromosomes and Genes
Article objectives
Genetics is the branch of biology that focuses on heredity. The basics of heredity are similar for all organisms that reproduce sexually: the offspring receive one set of genetic material from one parent and the other set from the other parent. But are there aspects of genetics that are specific for us?
A genetic disease is a phenotype due to a mutation in a gene or chromosome. Many of these mutations are present at conception and are therefore in every cell of the body. Mutant alleles may be inherited from one or both parents, resulting in a dominant or recessive hereditary disease. Currently, there are over 4,000 known genetic disorders, with many more phenotypes yet to be identified. Theoretically, every human gene, when disrupted due to a mutation, could result in at least one disease-type phenotype. Genetic diseases are typically diagnosed and treated by a geneticist, a medical doctor specializing in these disorders, many of which are extremely rare and difficult to diagnose. Individuals and families with genetic diseases, or suspected genetic diseases, are often counseled by genetic counselors, individuals trained in human genetics and counseling. To understand human genetic diseases, you first need to understand human chromosomes and genes.
The Human Genome
What makes each one of us unique? You could argue that the environment plays a role, and it does to some extent. But most would agree that your parents have something to do with your uniqueness. In fact, it is our genes that make each one of us unique – or at least genetically unique. We all have the genes that make us human: the genes for skin and bones, eyes and ears, fingers and toes, and so on. However, we all have different skin colors, different bone sizes, different eye colors and different ear shapes. In fact, even though we have the same genes, the products of these genes work a little differently in most of us. And that is what makes us unique.
The human genome is the genome - all the DNA - of Homo sapiens. Humans have about 3 billion bases of information, divided into roughly 20,000 genes, which are spread among non-coding sequences and distributed among 24 distinct chromosomes (22 autosomes plus the X and Y sex chromosomes) (Figure 1). The genome is all of the hereditary information encoded in the DNA, including the genes and non-coding sequences. The Human Genome Project has produced a reference sequence of the human genome. The human genome consists of protein-coding exons, associated introns and regulatory sequences, genes that encode other RNA molecules, and “junk” DNA, regions in which no function as yet been identified.

Figure 1: The Human Genome is depicted as the stained chromosomes at the top of the figure. The genome consists of chromosomes, which are composed of genes and other regions of DNA between the genes. Notice that there are 23 pairs of chromosomes.
Chromosomes and Genes
The human genome consists of 24 distinct chromosomes: 22 autosomal chromosomes plus the sex-determining X and Y chromosomes. A chromosome is a threadlike molecule of genes and other DNA located in the nucleus of a cell. Different organisms have different numbers of chromosomes. Human somatic cells have 23 chromosome pairs for a total of 46 chromosomes: two copies of the 22 autosomes (one from each parent), plus an X chromosome from the mother and either an X or Y chromosome from the father (Figure 2).

Figure 2: The human genome has 23 pairs of chromosomes located in the nucleus of somatic cells. Each chromosome is composed of genes and other DNA wound around histones (proteins) into a tightly coiled molecule.
There are an estimated 20,000 human protein-coding genes, but many more proteins. Most human genes have multiple exons separated by much larger introns. Regulatory sequences controlling gene expression are associated with exon sequences. The introns are usually excised (removed) during post-transcriptional modification of the mRNA. Human cells make significant use of alternative splicing to produce a number of different proteins from a single gene. So even though the human genome is surprisingly similar in size to the genomes of simpler organisms, the human proteome is thought to be much larger. A proteome is the complete set of proteins expressed by a genome, cell, tissue, or organism.
Linkage
As stated above, our roughly 20,000 genes are located on 24 distinct chromosomes. Linkage refers to particular genetic loci, or alleles inherited together, suggesting that they are physically on the same chromosome, and located close together on that chromosome. Two or more loci that are on the same chromosome are physically connected and tend to segregate together during meiosis, unless a cross over event occurs between them. A crossing-over event during prophase I of meiosis is rare between loci that usually segregate together; these loci will usually be close together on the same chromosome. They are, therefore, said to be linked. Alleles for genes on different chromosomes are not linked; they sort independently (independent assortment) of each other during meiosis.
A gene is also said to be linked to a chromosome if it is physically located on that chromosome. For example, a gene (or loci) is said to be linked to the X-chromosome if it is physically located on the X-chromosome chromosome. The physical location of a gene is important when analyzing the inheritance patterns of phenotypes due to that gene. The inheritance patterns of phenotypes may be different if the gene is located on a sex chromosome or an autosome.
Linkage Maps
The frequency of recombination refers to the rate of crossing-over (recombination) events between two loci. This frequency can be used to estimate genetic distances between the two loci, and create a linkage map. In other words, the frequency can be used to estimate how close or how far apart the two loci are on the chromosome.
In the early 20th century, Thomas Hunt Morgan, working with the fruit fly Drosophila Melanogaster, demonstrated that the amount of crossing over between linked genes differs. This led to the idea that the frequency of crossover events would indicate the distance separating genes on a chromosome. Morgan’s student, Alfred Sturtevant, developed the first genetic map, also called a linkage map.
Sturtevant proposed that the greater the distance between linked genes, the greater the chance that non-sister chromatids would cross over in the region between the genes during meiosis. By determining the number of recombinants - offspring in which a cross-over event has occured - it is possible to determine the approximate distance between the genes. This distance is called a genetic map unit (m.u.), or a centimorgan, and is defined as the distance between genes for which one product of meiosis in 100 products is a recombinant. So, a recombinant frequency of 1% (1 out of 100) is equivalent to 1 m.u. Loci with a recombinant frequency of 10% would be separated by 10 m.u. The recombination frequency will be 50% when two genes are widely separated on the same chromosome or are located on different chromosomes. This is the natural result of independent assortment. Linked genes have recombination frequencies less than 50%.
Determining recombination frequencies between genes located on the same chromosome allows a linkage map to be developed. Linkage mapping is critical for identifying the location of genes that cause genetic diseases.
Variation
As stated above, even though we essentially all have the same genes, the gene products work a little different in all of us, making us unique. That is, the variation within the human genome results in the uniqueness of our species. In fact, genetically speaking, we are all about 99.9% identical. However, it is this 0.1% variation that results in our physical noticeable differences, as well as traumatic events such as illnesses or congenital deformities. These differences can also be used for societies benefits, such as through forensic DNA analysis. Most studies of this genetic variation focus on small differences, know as SNPs, or single nucleotide polymorphisms, which are substitutions in individual bases along a chromosome. For example, the single base change from the sequence GGATAACGTCA to GGAAAACGTCA would be a SNP. Although not occurring uniformly, in the human genome, it has been estimated that SNPs occur every 1 in 100 to 1 in 1000 bases.
DNA sequences that repeat a number of times are known as repetitive sequences or repetitive elements. For example the sequence CACACACACACACA would be a dinucleotide (2 base) repeat, or the sequence GATCGATCGATCGATCGATC would be a tetranucleotide (4 base) repeat. The genomic loci and length of certain types of repetitive sequences are highly variable from person to person, which is the basis of DNA fingerprinting and DNA paternity testing technologies. Longer repetitive elements are also common in the human genome. Examples of repeat polymorphisms are described in Table 1.
Table 1 Repeat Polymorphisms (bp = base pair)
| Dinucleotide | repeats of two bp sequences |
|---|---|
| Tetranucleotide | repeats of four bp sequences |
| Microsatellite; Short Tandem Repeats (STRs) | short sequences of 100-200 bp, usually due to repeats of 1-6 bp sequences |
| Minisatellite | short sequences of 6-10 bp repeats |
| VNTR: Variable Number of Tandem Repeat | short nucleotide sequence ranging from 14 to 100 nucleotides long, organized into clusters of tandem repeats, usually repeated in the range of between 4 and 40 times per loci |
Autosomes and Sex Chromosomes
There are 44 autosomes and 2 sex chromosomes in the human genome, for a total of 46 chromosomes (23 pairs). Sex chromosomes specify an organism’s genetic sex. Humans can have two different sex chromosomes, one called X and the other Y. Normal females possess two X chromosomes and normal males one X and one Y. An autosome is any chromosome other than a sex chromosome. Figure 3 shows a representation of the 24 different human chromosomes. Figure 4 shows a karyotype of the human genome. A karyotype depicts, usually in a photograph, the chromosomal complement of an individual, including the number of chromosomes and any large chromosomal abnormalities. Karyotypes use chromosomes from the metaphase stage of mitosis.
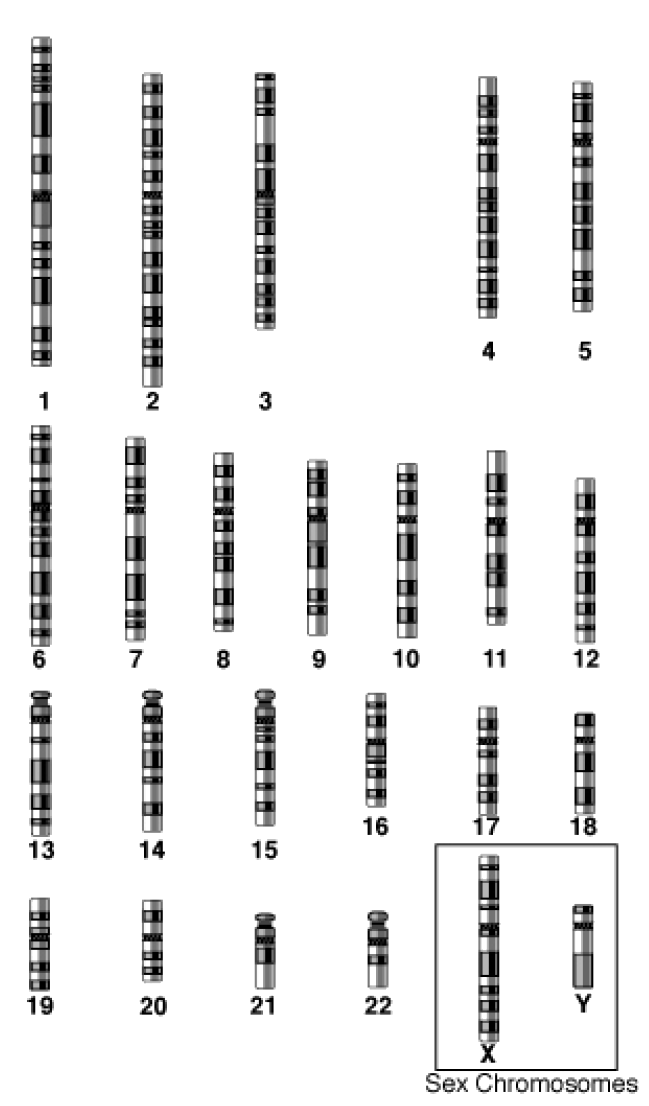
Figure 3: The 24 human chromosomes. The autosomes are numbered 1 - 22, based on size, with chromosome 1 being the largest. The X and Y sex chromosomes are shown in the box.

Figure 4: A karyotype of the human genome. Is this from a male or female?
The 22 autosomes are numbered based on size, with the largest chromosome labeled chromosome 1. These 22 chromosomes occur in homologous pairs in a normal diploid cell, with one of each pair inherited from each parent. The sex of an individual is determined by the sex chromosome within the male gamete. Females are homologous, XX, for the sex chromosomes, whereas males are heterozygous, XY. As all individuals inherit an X chromosome from their mother (females can only produce gametes with an X chromosome), it is the sex chromosome that they inherit from their father that determines their sex.
Sex-Linked Genes
Sex-linked genes are located on either the X or Y chromosome, though it more commonly refers to genes located on the X-chromosome. For that reason, the genetics of sex-linked (or X-linked) diseases, disorders due to mutations in genes on the X-chromosome, results in a phenotype usually only seen in males.
In humans, the Y chromosome spans 58 million bases and contains about 78 to 86 genes, which code for only 23 distinct proteins, making the Y chromosome one of the smallest chromosomes. The X chromosome, on the other hand, spans more than 153 million bases and represents about 5% of the total DNA in women’s cells, 2.5% in men’s cells. The X chromosome contains about 2,000 genes, however few, if any, have anything to do with sex determination. The Y chromosome is the sex-determining chromosome in humans and most other mammals. In mammals, it contains the gene SRY (sex-determining region Y), which encodes the testes-determining factor and triggers testis development, thus determining sex. It is the presence or absence of the Y chromosome that determines sex.
X-Inactivation
Early in embryonic development in females, one of the two X chromosomes is randomly inactivated in nearly all somatic cells. This process, called X-inactivation, ensures that females, like males, have only one functional copy of the X chromosome in each cell. X-inactivation creates a Barr body, named after their discover, Murray Barr. The Barr body chromosome is generally considered to be inactive, however there are a small number of genes that remain active and are expressed.
Images courtesy of:
http://en.wikipedia.org/wiki/Image:Human_genome_to_genes.png. CC-BY 2.0.
http://www.genome.gov/Pages/Hyperion/DIR/VIP/Glossary/Illustration/chromosome.cfm?key=chromosome. Public Domain.
http://www.genome.gov/Pages/Hyperion/DIR/VIP/Glossary/Illustration/karyotype.cfm?key=karyotype. Public Domain.